Describe the Arrangement of Sodium Ions and Chloride Ions
These ionic bonds form when the. Why do ionic compounds conduct conduct electric current when they are melted or dissolved in water.
9 4 Ionic Bonding Chemistry Libretexts
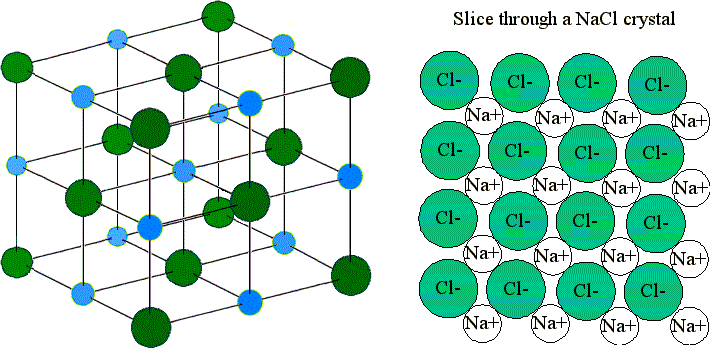
Adapted from Chemguide A sodium ion sits at the centre of the cube and its nearest neighbours are the chloride ions at the centre of each of the six faces.

. Sodium atom has only 1 electron in its outermost shell. The most common use of sodium chloride is in the Solvay process for the production of sodium carbonate and calcium chloride. In the crystal lattice each cation of sodium is surrounded by six chloride anions and each chloride ion is in turn surrounded by six sodium ions.
To do this use the following variables and make these assumptions. The sodium ion in the centre is being touched by 6 chloride ions. The ions in solid sodium chloride are arranged in an orderly pattern.
Inside the crystal lattice each sodium ion Na1 is surrounded by six chloride ions Cl1- and each chloride ion is surrounded by six sodium ions. Write the name and symbol of the ion formed when. The combined weight of the pilot basket together with that of the balloon fabric and other.
Explanation of formation of sodium chloride. A calcium atom loses two electrons. Name two widely used alloys and describe some of their uses.
It appears as a white crystalline solid and is usually odorless in nature. A Describe the arrangement of the ions and the type of attractive forces between the ions in solid magnesium chloride. In crystal structure of sodium chloride the arrangement of Cl ion is.
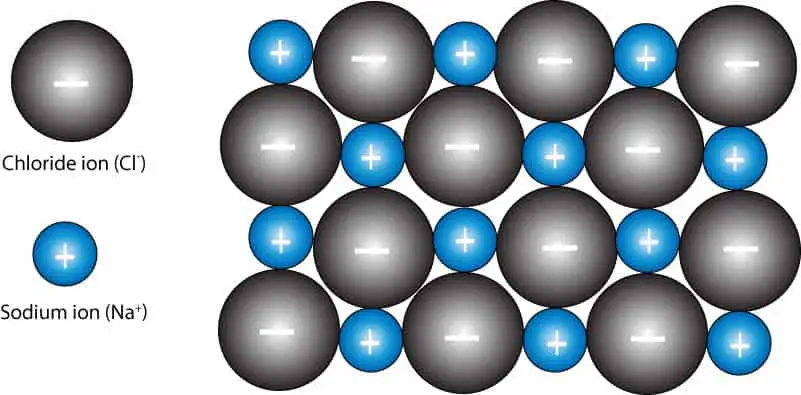
The electrons are able to move freely around in the metal making them more susceptible to. The ions in a compound such as sodium chloride are arranged in a lattice structure. How can you describe the arrangement of sodium ions and chloride ions in a sodium chloride.
By chance we might just as well have centred the diagram around a chloride ion - that of course would be touched by 6 sodium ions. It is a crystalline solid containing positive sodium ions surrounded by six chloride ions and vice versa. A2 Sodium chloride NaCl and magnesium chloride MgCl 2 are both ionic compounds.
In crystal structure of sodium chloride the arrangement of. These ions are held together by strong electrovalent bond. The sodium chloride crystal structure consists of a face-centred cubic lattice.
The diagram shows part. In this way a positive sodium ion Na and a negative chloride ion Cl-. The atomic number of chlorine is 17 so its electronic configuration is 2 8 7.
An aluminum atom loses three electrons. It forms an alternating regular and repeating three dimensional pattern. How can you describe the arrangement of sodium ions and chloride ions in a crystal of sodium chloride.
A sulfur atom gains two electrons. The sodium and chloride ions in a crystal of sodium chloride form a crystal lattice. Remember that the lattice arrangement is giant - for example a single grain of.
This regular arrangement results in the formation of a crystal. Chloride ions are ccp type of arrangement ie it contains. Each sodium ion is touched by 6 chloride ions.
Only those ions joined by lines are actually touching each other. Hot air balloons float in the air because of the difference in density between cold and hot air. Sodium chloride also known as salt or halite is an ionic compound with the chemical formula NaCl representing a 11 ratio of sodium and chloride ions.
A two-dimensional model for the ionic lattice in sodium chloride. The attractive force between sodium ions and chloride ions results in an arrangement of ions in repeating units arranged to form a _____ crystal lattice. In this problem you will estimate the minimum temperature the gas inside the balloon needs to be for it to take off.
In the sodium chloride crystal arrangement the net effect is that the. Each sodium ion is touched by. Sodium chloride is a giant ionic lattice consisting of alternating adjacent positively charged sodium ions and negatively charged chloride ions in a regular arrangement of rows and columns.
In the ionic bonding of Sodium Chloride NaCl the sodium atom loses one electron to become a cation and the chlorine atom gains one electron to become an anion. The array of ion merge together to form a face centered cubic structure. With molar masses of 2299 and 3545 gmol respectively 100 g of NaCl contain 3934 g Na and 6066 g Cl.
Ions next to each other. The salient features of its structure are. The atomic number of sodium is 11 so its electronic configuration is 2 8 1.
The arrangement of ions in sodium chloride shows that each ion is surrounded by _____ oppositely- charged ions. A nitrogen atom gains three electrons. The structure of sodium chloride is in a lattice shape and is described as having a giant ionic structure.
So the sodium atom donates 1 electron and forms a sodium ion Na. Sodium chloride is described as being 66-co-ordinated. The structure of sodium chloride is in a lattice shape and is described as having a giant ionic structure.
Sodium chloride composed of two ions Na and Cl- is an ionic compound having the chemical formula NaCl. The movement of ions allows electric current to flow between the electrodes through an external wire. The ions are held together in the lattice by strong ionic bonds between the oppositely charged sodium and chloride ions.

Ionic Crystal Structures Chemistry For Non Majors
No comments for "Describe the Arrangement of Sodium Ions and Chloride Ions"
Post a Comment